Heat is not a substance but rather energy that can be gained or lost. Early scientist thought that heat could just evolve and naturally flow between hot and cold objects. Benjamin Thompson (Count Rumford disproved this by showing that as you drilled the hole in cannon barrels under water the temperature of the water rose. He concluded that the temperature rise in the water was due to the work that was being done on the cannon and that is was not just flowing by itself. He concluded that heat must be a form of energy.
All matter is made up of very tiny individual particles called atoms or molecules. The way these particles are arranged determine whether they are a solid liquid or gas. In a solid the particles are in a fixed position but allowed to vibrate. In a liquid they are not bonded in a fixed position but they are very close together and allowed to slide past one another. In a gas they are spread far apart and not bonded at all. The vibrations, movements, and spacing of these particles determine the amount of thermal energy that a substance has.
Heat is the transfer of this thermal energy from one substance to another. Imagine that you have a group of people standing together in a group. If you have another group of students run into the first group what would happen to the first group of students? They would move faster and further apart. This is exactly what happens whey you bang the two ball bearings together and produced the burn mark on the piece of paper. As the particles of a substance vibrate and move faster, or farther apart, they gain energy. Heat moves from hot to cold. Therefore cold really means the absence of heat. You cannot add cold.
Thermal energy: Is the total energy of all the atoms or molecules that make up a substance. This includes the kinetic energy of the particles (how fast they are moving) and the potential energy of the particles (how far apart they are) Gases have more potential energy than liquids. Why?
Thermal energy can be increased by increasing either the kinetic or potential energy of the particles. That is why something gets hot due to friction. By rubbing the two objects together you are causing the particles to vibrate at a faster pace and thus it increases their thermal energy..
Thermal energy can also cause the expansion of a material. Remember when we had the ball and hoop of metal that we stuck in the flame! Almost all materials will expand when heated. This is why we need to cut expansion joints in concrete and also have expansion joints at the ends of bridges. Also remember how the bimetallic strip worked in class and how this process can be used as a thermostat.
What about expansion in water? Water also expands when heated but does NOT expand when heated from 0-4 degrees Celsius. See discussion on page 146 of you text.
Temperature is a measure of thermal energy. It tells us how hot or cold an object is . Temperature is measure by thermometers which measure the expansion or contraction of a liquid. In class remember we made water thermometers. Thus temperature measures the AVERAGE kinetic energy of the particles!!
Temperature scales
| phase change | Celsius | Fahrenheit | Kelvin |
| freezing | 0 | 32 | 273 |
| boiling | 100 | 212 | 373 |
Remember O degrees Kelvin (-459 F) is absolute zero. This is the temperature at which there would no more kinetic energy in the particles thus it would not have any temperature. If this substance was a gas it would also have 0 pressure because the molecules would not be moving. Kelvin is a useful scale because there are no negative numbers in Kelvin.
Heat: Is the movement of thermal energy from one substance to another. Remember there are three ways to move thermal energy.
1.) Conduction: This happens when two objects are in direct contact. When you touch a hot stove you gain heat by conduction. Think of a jar full of ping-pong balls. When you push on the ping pong balls on the top, the energy is transmitted through all of the balls all of the way to the bottom of the jar. When you touch a hot stove the particles making up the burner are vibrating very fast. They collide with the particles of your finger making them vibrate and thus the energy is transferred. Some substances are capable of transmitting these vibrations very quickly. The are called conductors. Most metals are good conductors. Insulators are not able to transmit this energy very efficient. These are called insulators. Glass, wood, and plastics are generally good insulators. Can you see now why some frying pans have wooden or plastic handles?
2.) Convection occurs in liquids and gases. Convection is similiar to conduction in the fact that molecules transfer the energy from one substance to another. However in liquids and gases the molecules are allowed to move. Therefore as molecules gain energy this part of the fluid rises and as the lose energy the fluid sinks. This rise and sinking of air or water sets up convection currents within the fluid. Watch this video of an ice cube in warm water. Notice the cold water sinking underneath the ice cube.
3.) Radiation is the transfer of energy without the use of molecules. In other words it is radiant energy that is just absorbed by a substance. It does not need matter to transfer the energy. This is the way the sunlight warms the earth. There is no air in space yet the temperature of the earth is increased by sunlight. Radiant heat is responsible for this.
Cold: Is the absent of heat. You CANNOT add cold!! You can only remove heat.
Heat is either measured in calories or joules. A calorie is the amount of energy needed to raise 1 gram of water 1 degree C. (1 calorie=4.18 J)
If you have 50 grams of water that goes from 10 degrees C to 40 degrees C, how many calories of heat were absorbed by the water?
Food is measured in kilocalories or Calories. Thus 1000 calories = 1 kilocalorie = 1 Food Calorie.
The three laws of thermodynamics:
1.) Whenever heat flows into out of a system, the gain or loss of thermal energy equals the amount of heat transfer.
If you put a can of warm pop in the refrigerator the heat from the can goes into the air of the fridge. However the heat lost by the can must always equal the heat gained by the air of the fridge. Can you see why?
If you heat up a confined gas so that it moves the piston. The amount of work that can be done by the movable piston cannot be greater than the heat energy that you put into the gas.
2.) Heat never spontaneously flows from a cold substance to a hot substance. If you open your door during the winter the heat goes from the inside to the outside. Again you cannot add cold. Some of you who have a heat pump at home should investigate how a heat pump works.
3.) No system can reach absolute zero. Why?
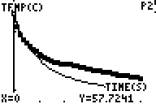
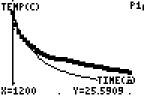
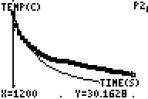
Specific heat: Do all materials heat and cool at the same rate? Look at the following experiment
I put one test tube of oil and one test tube of water into some hot water until both there temperatures were the same (around 57.7 degrees ) The mass of the oil and the mass of the water were the same. (16 grams for each)



( initial temps are the same) (final temp oil) (final temperature water)
Questions:
1.)
Which substance had the greatest temperature change?
Does this mean they lost heat at the same rate or one of them lost heat
faster? Explain.
2.) Think back to class and what were the two factors that we said accounted for heat? (mass and change in temperature). However, if these were the only two factors then they should cool at the same rate. There must be something else going on here. That is some substances will naturally cool faster than others. This is called the specific heat of a substance (page 141)
Specific heat: Amount of energy needed to raise 1 gram of a SUBSTANCE 1 degree Celsius.
Remember a calorie is the amount of energy needed to raise 1 gram of WATER 1 degree Celsius .
Notice they are very similar but one is for a the particular substance and one is for water.
3.) Each substance is unique in its ability to gain heat. This property is called the specific heat of a substance. You may have notice that some things such as metals tend to warm up very quickly while other substances such as water take a long time to heat up. This is due to the fact that they have different specific heats. Iron's specific heat is .11 cal/g-degree while water is 1.00 cal/g-degree. This means that it takes about ten times the amount of energy to heat up the same mass of water as it does for the same mass of iron! Here is a table of specific heats.
Think about your house and a wood or tile floor vs. a carpeted floor. Which floor is colder? Explain. Which feels colder? Explain.
Why went I go outside does it feel like the flagpole is colder than the ground? Is it or not? Explain.
4.)
Sample problems: (specific heat of water is 1 cal/g-C and iron is .11 cal/g-C
1.) You have 300 grams of water at 60 degrees C. It cools to 20 degrees C. How many calories of heat did it lose?
2.) You have 300 grams of iron at 60 degrees C. It cools to 20 degrees C. How many calories of heat did it lose?
Lab: Mix it up
Lab: How many calories can you get from a match
Does windchill affect nonliving substances? Why or why not?
Chapter 10 Notes
Review Heat transfer by Conduction, convection, and Radiation as outlined in the notes above
Remember conduction is from touching an object and primarily through electron vibrations. Insulators are objects that do not allow their electrons to move or vibrate and thus they do not conduct heat very well.
Convection is heat transfer in liquids and gases. It is caused by different densities in the substances which is caused by different temperatures within the substance.
Radiation: does not need air or liquid to travel through. It is energy in the form of an electromagnetic wave that is emitted by an object.
Remember the parts of the electromagnetic spectrum that we discussed in class!
Remember the longer the wavelength the lower the frequency and thus the less energy the wave has. Low temperature objects emit long wavelengths.
The shorter the wavelength, the higher the frequency, and thus the more energy the wave has. High temperature objects emit short wavelengths.
All objects will absorb radiant energy. Therefore all objects will emit radiant energy. A good absorber (black) is also a good emitter. Therefore a black object will heat up first in the sun, and a dark object will lose heat faster at night.
Remember if an object is hotter than its surroundings it is an emitter and if it colder it is an absorber.
No object is a perfect absorber. Some radiant energy is always reflected. Some colors and able to reflect more than others. Remember our lab at the beginning of the year?
How can a substance change its phase?
1.) Change its temperature: This causes the molecules to slow down or speed up
2.) Change the pressure: This moves the molecules closer together or farther apart.
Evaporation: Is a surface phenomenon. It is the changing of a liquid into a gas (physical change) It only happens at the surface of a liquid and can happen at any temperature above the freezing point. It is a cooling process!!
Changes of state
| Name | Definition | Energy change | Particle arrangement |
| Melting | solid to a liquid | gains | fixed to be able to slide |
| Freezing | liquid to a solid | loses | slide to a fix |
| condensation | gas to a liquid | loses | unattached and far away to attached, close and slide |
| vaporization | liquid to a gas | gains | attached, close and slide to unattached and far away. |
| sublimation | solid to a gas | gains | fixed and close together to unattached and far away |
| Deposition | gas to a solid | loses | unattached and far away to close together and fixed |
Heat of vaporization : amount of energy needed to change one gram of liquid to one gram of gas. For water the it is 540 cal/grams
Heat of fusion: amount of energy needed to change of a solid to 1 gram of a liquid. For ice it is 80 cal/gram.
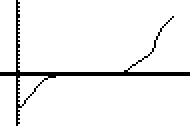

Which one is the freezing of water and which one is the melting of ice? What is happening at the flat portion of the line?
|
|
|
|
Page Last Updated: Monday November 19, 2001 Webmaster: Larry Jones Pickens County School District |
taken from http://www.sciencebyjones.com/phase_change_diagram.htm